The buffering mechanism consists of two reversible reactions where the . Its chemical structure contains a morpholine ring. Tris buffers are used commonly in microtechnique applications involving molecular biological procedures. Henderson-Hasselbalch equation to get a lot of different With a pKa of 7.20, MOPS is an excellent buffer for many biological systems at near-neutral pH. Proteins are covalently coupled with a blue chromophore and, different cell types. Plant Microtechnique and Microscopy, Use TBS when performing immunocytochemical, experiments on phosphate-sensitive tissues, Tris base DI Dissolve and adjust pH with the following approximate amount of HCl: pH 7.4 pH 7.6 pH 8.0, Disodium ethylene diamine tetraacetate Adjust pH to approx. talk about the relationship between pH and pK_a and buffers. Mix the three purified enzyme components of NBDO in 50 mM MES buffer at pH 6.8 containing 100 M (NH 4) 2 Fe(SO 4) 2 to final concentrations of 0.3 M reductase, 3.6 M ferredoxin, and 0.3 M oxygenase.. 2. I forgot a minus sign there. what happens to the buffer ratio if the pH is raised? Metal binding: (Compare with other buffers), Solubility in water:(Compare with other buffers), Saturation at 0C:(Compare with other buffers), Copyright 2023 HOPAX. MES is not a good buffer for pH 4 because the pKa of MES is 6.1. Optimize Reagents MES (buffering agent) Morpholinoethanesulfonic acid 2- (4-morpholinyl)-1-ethanesulfonic acid 2- (4-Morpholino)ethanesulfonic acid Morpholino-N-ethylsulfonate Ethanesulfonic acid, 2-morpholino- 2- (morpholin-4-yl)ethanesulfonic acid NSC 157116 2- (N-Morpholino)ethansulfonsaeure 2- (N-Morpholino)aethansulfonsaeure MFCD00006181 So the Henderson-Hasselbalch equation just says that the pH is equal to the pK_a plus the log of A minus over HA, where HA is our weak acid and A minus is its conjugate base. So just to wrap up, we can look at the Henderson-Hasselbalch equation and we can just look at the relationship between pK_a and pH, and depending on whether Stock solutions are. Dissolve the nitroaromatic substrate (e.g., nitrobenzene) in MES buffer and add an appropriate volume to the enzyme mixture to give an initial substrate concentration of 200 M. We consider H2O(l) as 1 because it is not going to be relevant in finding our pH or pOH. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Generally, the pH value is set using NaOH/ . ,PLPPAc26LF)aw0\-3-[s"2H1AL1O1 H1OJ1OJ1)L1M1f_O|9_8b_\XEVyqlh^DmEHs^V%xv!"'-(Bh"osD3@A @BY!6L#DB:k8h(gq~"dZuy]?F=S Language links are at the top of the page across from the title. Add the following amounts of 0.2 M HCl per 100 ml cacodylate stock solution, followed by the addition of DI to a final volume of 400 ml, to obtain 0.05 M cacodylate buffer at the desired pH (Dawes, 1971). All rights reserved. Components: MES Monohydrate(1 M), Water(rest) Method: Prepared in 18.2 megohms-cm 1 water and filtered through 0.22-micron filter. In personal care and cosmetics applications, MES can protect the active ingredients of the formulations against degradation. relative relationship and size between A minus and HA concentration. Crystal structures of mutant Pseudomonas aeruginosa p-hydroxybenzoate hydroxylases: the Tyr201Phe, Tyr385Phe, and Asn300Asp variants. Import fees:the customer must pay these fees directly to the delivery company (DHL) when receiving the product. 1. and easy way of doing that. Since protonation alters the overall molecular charge, and can subsequently affect the structure of a molecule, most biological molecules are only functional in a certain pH range, Alternatively, volumes of equimolar MES free acid and sodium MES can be mixed to attain the desired pH. concentration is always one. . It has the advantages of: midrange pKa, maximum water solubility and minimum solubility in all other solvents, minimal salt effects, minimal change in pK with temperature chemically and The other thing that you can use the Henderson-Hasselbalch equation to tell you is the relationship between A minus and HA which is something you might wanna know. I usually just remember Direct link to Jonathan Acosta Hernandez's post We consider H2O(l) as 1 b, Posted a year ago. Copyright 2006-2023 Thermo Fisher Scientific Inc. All rights reserved, Spectroscopy, Elemental and Isotope Analysis, Protein Electrophoresis & Western Blotting, Compare protein migration patterns using MES and MOPs on NuPAGE Bis-Tris gels, See all available buffers and reagents available for SDS-PAGE. [1] MES is also useful as a non-coordinating buffer in chemistry involving metal ions, as many common buffers (e.g. Which tells us that this ratio is equal to one. Products > So anything to the zeroth power is equal to one. 2/22/2021 8:55:39 AM. So if your pH is bigger than your pK_a, then this term up here, 10 to the pH minus pK_a, Solution: The Henderson-Hasselbalch equation, which links the pH of a buffer solution to its acid dissociation constant (pKa) and the ratio of the concentrations of the conjugate base (A-) and acid (HA) forms of the buffer, must be used to calculate the pH of a 0.35 M MES buffer. To 50 ml of 0.2 M sodium barbital (Veronal. pK_a for your buffer. Any time you have a buffer and the pH of your solution is equal to the pK_a of your buffer, you immediately know If the product you are interestedis available only in bulk quantities, click on "Request a Quotation". because it's a pure liquid, so we assume that the H+ then reacts with water to form H3O+. MES was developed as one of 'Good' stamps in the 1960s. 4 0 obj Components: MES Monohydrate(500 mM), Sodium Hydroxide(for pH adjustment), Water(Rest) Method: Prepared in 18.2 megohms-cm 1 water and filtered through 0.22-micron filter. This tells us that the reaction is essentially irreversible, or it only goes in one direction; towards the products with very little reactants reformed in the reverse direction. 3 0 obj that pH equals pK_a means you have the same concentration, and then, if I forget, I will We can construct an acid dissociation expression for strong acids and calculate their Ka and pKa values. MOPS (3-(N-morpholino)propanesulfonic acid) is a buffer introduced in the 1960s, one of the twenty Good's buffers. Direct link to Amanda Saideepane's post If exactly half the amoun, Posted 2 years ago. We are sad to see you go. 1PF&P@nMQDDv$ ~k {,j3,vY"ZX kH|{/rOr8 sX+G(ux~z}7mH;#G{K=5W?77|wkk8\dn^54n?7y6q7?S/O>`}@Lgwvfy~_Q8K|7IRn_Yn,lo!7|w-t_P[?9Y|n^xB.WFG^;=^is2]%7Nzou uI#vl+cq};HslxG#qS8 eJyQ}EpVd S;Zufhx4Fq?legnL{bEkLC0ZKZY/#y!_MY\)k{?lW10SA:tW_9c_\z]kj/M7#5[@vRwwe}F'Ke\XnqW}C6~uw%w\ KTpQdpO7cug?WOvn_[.lUWRcTXQdokka15ot_a.8o][2Q^Iyr'5~@0J,vow c\/}R}e(F8,oSS\f.?BNdp.]cq'}LBbo!dbo8l=V/B;EvU5K{|Ruk0Mosl{_js6~x}6v;0c9n=I.;Z_?+0h_:GZ")o,{`\pq] Not Intended for Diagnostic or Therapeutic Use. MOPS (CAS 1132-61-2), 3-(N-Morpholino)Propanesulfonic Acid. So we're gonna subtract pK_a from both sides and that gives us a log of A minus over HA. pKa values describe the point where the acid is 50% dissociated (i.e. Below are tables that include determined pKa values for various acids as determined in water, DMSO and in the gas Phase. what the relationship is between our acid and its Listed here are a number of common Tris formulations (Maniatis, Combine 25 ml glycine stock solution with, University of California, Berkeley Rausser College of Natural Resources, Zeiss/Yokagawa Spinning Disk confocal microscope, Zeiss Lumar epifluorescence stereo microscope, Ruzin, 1999. When MES solutions are autoclaved, they turn yellow (although pH does not change measurably). CITRATE BUFFER; PH 3.06.2, PKA = 6.40 Citrate buffer (Gomori, 1955) stock solutions: A: 0.1 M citric acid; B: 0.1 M sodium citrate. And this comes up a lot not Terms & Conditions, By submitting your email address, you are agreeing to our, MES hydrate free-flowing, Redi-Dri(TM), >=99.0%. (5) Complex with organotin (IV) molecules as antitumor agents. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. And I don't know about Posted 7 years ago. These values indicate that the products of a strong acid reaction are heavily favored compared to the reactants. So pKa is equal to 9.25. It's something that Thanks! Expert Answer. Calculate the composition of buffer solution containing CH3COOH. Standard mixing tables using stock solutions to prepare a buffer of a given pH have been published. You want to make 1 L of a 1 M solution of MES (pH 6.2). that the concentration of your acid and its Oh, sorry, if you haven't I feel that you're correct. The Henderson-Hasselbalch equation gives you a really quick HEPES Sodium, used in conjunction with HEPES, is the most commonly used biological buffer formulation since the pKa of the buffer is 7.31 which corresponds to the physiological pH of 7.36. So there are two other possibilities for pH and pK_a. 2)The cart is displayed in the top right side of the page. For information about other types of mops, see, 3-(Morpholin-4-yl)propane-1-sulfonic acid, InChI=1S/C7H15NO4S/c9-13(10,11)7-1-2-8-3-5-12-6-4-8/h1-7H2,(H,9,10,11), InChI=1/C7H15NO4S/c9-13(10,11)7-1-2-8-3-5-12-6-4-8/h1-7H2,(H,9,10,11), Except where otherwise noted, data are given for materials in their, https://bostonbioproducts.com/products/mops-buffer-1-m-ph-74-bbm-74, https://en.wikipedia.org/w/index.php?title=MOPS&oldid=1132322434, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Chemical articles with multiple PubChem CIDs, Articles with changed ChemSpider identifier, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 8 January 2023, at 09:26. This article is about the biochemical buffering agent. The melting point is approx. Hopax is a manufacturer and supplier of MES (CAS 4432-31-9), a zwitterionic biological buffer often used as a buffering agent in biological and biochemical research. If exactly half the amount of a weak acid in solution is ionized at some pH, say 5, how do you find the pKa of the weak acid if you don't know anything else? High concentrations of MES are toxic to most plants, but can be used in plant media at . Taiwan Hopax Chemicals Mfg. Lah, MS; Palfey, BA; Schreuder, HA; Ludwig, ML. There are many variations. So if you know the pH and you know it's bigger MES interacts with peptide backbone of bovine serum albumin leading to a net stabilization of the protein 11. Also known as [2-(N-Morpholino) ethanesulfonic Acid]. Then what you said would be right. MES Buffer, 2-Morpholineethanesulfonic Acid. to a positive number, when you raise 10 to a positive number, you get a ratio that is greater than one. Used in different types of chromatography, such as gel-filtration, phosphocellulose column, hydrophobic interaction and cation exchange chromatography. Autoclaving is not recommended for any sulfonic acid buffer. is going to be positive. MES is a zwitterionic buffer. Buffer Table Crystal Growth 101 page 1 Buffer . Optimize - Buffers It has good pH buffering capacity within the range of pH 5.07.4. 300C. to get rid of the log by raising both sides to the 10th power. The melting point is approx. Biochemistry 33, 1555- 1564, 1994. Which tells us that this HEPES Sodium is considered to be non-toxic to culture cell . pKa: 6.1. MES monohydrate; CAS Number: 145224-94-8; Synonyms: 2-(N-Morpholino)ethanesulfonic acid,4-Morpholineethanesulfonic acid monohydrate; find Sigma-Aldrich-163732 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich It is a structural analog to MES,[1] and like MES, its structure contains a morpholine ring. You weigh out 1 mole of the basic (unprotonated) form of MES and dissolve it in 750 ml water. If you are sure about your decision, please writedown your e-mail and click onunsubscribe. Since this Good's Buffer lacks the capability to form a complex with most metal ions, therefore it is recommended for use as a noncoordinating buffer in solutions with metal ions. Language links are at the top of the page across from the title. So we're gonna plug that into our Henderson-Hasselbalch equation right here. > MES monohydrate Buffer, Synonyms: MES or 2-(N-morpholino)ethanesulfonic acid or 4-morpholineethanesulfonic acid monohydrateC6H13NO4S H2OMr 213.25CAS Number [145224-94-8]EC Number 224-632-3Beilstein Registry Number 329824236RTECS QE3479500MDL Number MFCD00149409Purity 99.5%pKa 6.1 at 25C. Use x ml A + y ml B and dilute to 100 ml with 50 ml DI. Used for mammalian cell culture 8. Based on this expression K_a is just equal to H_three O plus times A minus all over the concentration of HA, and we don't include water This is a really helpful 300 C. The identity of the yellow breakdown product is unknown. MES, Free Acid, ULTROL Grade Write a review A zwitterionic buffer useful in the pH range of 5.5-6.5. Cacodylate was introduced for electron microscopy applications by Sabatini. Direct link to Ramisha Ghazi's post Calculate the composition, Posted 2 years ago. Buffer solution is used in biochemical experiments to stabilize the pH of the system. endobj [2] MES is highly soluble in water. endobj One of the "Good" buffers developed for biological applications. 4)Write down the buyer and shipping information. And then that means you From Plant & Animal Microtechnique, SE Ruzin, Oxford Univ Press, 1999, From Ruzin, 1999. about buffers, that's okay. acid sodium salt Formula: C6H12NNaO4S Formula Weight: 217.22 CAS No. The pH (and pKa at ionic strength I0) of the buffer solution changes with concentration and temperature, and this effect may be predicted using online calculators. please read on, stay posted, subscribe, and we welcome you to tell us what you think. Application: MES, Sodium Salt is a buffering agent used in biological and biochemical research including plant cell culture CAS Number: 71119-23-8 Purity: 99% Molecular Weight: 217.22 Molecular Formula: C 6 H 12 NO 4 SNa For Research Use Only. So if our ratio A minus over HA is greater than one, that tells us that A minus, the numerator, Identify which Good's buffers are most effective near pH 7.0 and near pH 6.0. Titration 1. Terms & Conditions, By submitting your email address, you are agreeing to our, Murashige & Skoog Medium with MES Buffer & Vitamins, MES-buffered saline (5X, pH 7.4), low endotoxin, ROCHE DOTAP Liposomal Transfection Reagent, N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N, ROCHE DOTAP Liposomal Transfection Reagent, N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate. It is recommended for separating small- to medium-sized proteins. MES is a popular choice for buffered culture media for bacteria, yeast and mammalian cells. It also emits toxic fumes upon combustion, including carbon monoxide, nitrogen oxide and sulfur oxides. other videos introducing it and also deriving it, For Research Use Only. Henderson-Hasselbalch equation, which I am gonna assume [2] MES is highly soluble in water. 0.5Na Formula Weight: 206.73 CAS No. conjugate base are the same. Absorbance (1.0 M, H2O, 260 nm):0.05. <> : 71119-23-8 Purity: 99% (titration) Other Notes: Easily compare specifications for MES sodium salt products with the MES sodium salt specification table . : 117961-21-4 Reconstitution: Contents of each pouch dissolved in 1 liter deionized, PAGEmark Blue PLUS Protein Markers A blue protein standard with 12 prestained proteins covering a wide range of molecular weights:10-240kDa in Tris-Glycine buffer and9-235kDa in Bis-Tris (MOPS) buffer or Bis-Tris (MES) buffer. to solve for this ratio that we might be interested in. Suitable for most toxicity studies 9. Direct link to s.m.guillaume's post at 1:32, correct me if I', Posted 7 years ago. Use our interactive chart below to compare the useful pH range of this product with other buffers. Further reading: How to choosing the biological buffer you need: By ph and pKa. So the pH of our buffer solution is equal to 9.25 plus the log of the concentration of A minus, our base. It is a zwitterionic, morpholinic buffer that is useful for a pH range of 5.5 - 6.7 and commonly used for cell culture media, as a running buffer in electrophoresis, and for protein purification in chromatography. It has minimal UV absorption and is often used in plant cell cultures and as a running buffer for bis-tris gels. actually less than one. SODIUM ACETATE; PH 3.6-5.6, PKA = 4.76 Good's buffers are compounds that are useful near neutral pH. &FubS-MQdNVH5e5 C(]]`/\pctf.j87 =qRKO5M-*Ou&\oXMNTQ}bY!8#)Z,y^\`'c3Dw~WcWMX]O r Lq$Hcp In water, HA can dissociate into A- and H+. HR2-943-07 - MES monohydrate pH 5.8 - 1.0 M solution is a Custom Shop reagent and is used to reproduce and optimize Crystallization Reagents for use with the Silver Bullets kit. just when you're talking about buffers by themselves, but also when you're doing titrations. wanna do to your solution, if you wanna add things to it, maybe you wanna add some Direct link to Khan academy's post No, as the 10 is added du, Posted 6 years ago. How the Henderson-Hasselbalch equation can be used to look at the ratio of conjugate acid and base using relationship between buffer pH and pKa. capacity of a specific buffer to be the pKa 1. Compound name: MES buffer. thing to remember. Synonyms: MES or 2-(N-morpholino)ethanesulfonic acid or 4-morpholineethanesulfonic acid monohydrate C 6 H 13 NO 4 S H 2 O M r 213.25 CAS Number [145224-94-8] EC Number 224-632-3 Beilstein Registry Number 329824236 RTECS QE3479500 MDL Number MFCD00149409 Purity 99.5% pKa 6.1 at 25C. And it just has a special If you still have any questions, feel free to contact us at. And I said really a lot there. pH: 6.0 0.15 Storage: 2-8C, Thomas Scientific 2023 All Rights Reserved. The buffer range is 5.5~6.7, pKa value of MES buffer is near to physiological pH, it is used in the following experiments: (1) Replace highly toxic cacodylate, and ion buffer citrate and malate; (2) Buffered media for bacterial, yeast and mammalian cells. 3FV4ZrPmLRhbiM}~^RiJc0;SMa Plant Microtechnique and Microscopy, CACODYLATE BUFFER; PH 5.07.4, PKA = 6.27. MOPS ( 3- (N-morpholino)propanesulfonic acid) is a buffer introduced in the 1960s, one of the twenty Good's buffers. The choice of buffer is not only according to the required buffer range and capacity of buffer solution, but also according to the specific experimental content. The pka of MES is 6.2. I use MES hemisodium salt for the buffer. contains both a weak acid, which generically we write as HA, and it also contains in equilibrium the conjugate base of So very large numbers for Ka and negative values for pKa for strong acids. MES; Common buffer compounds used in biology; References This page was last edited on 7 February 2023, at 00:31 (UTC). (4) Make the volume to 1L, then filter with 0.45m filter membrane to remove impurities, store at room temperature and protect from light. 8.0 and stir until dissolved, NaCl NaCitrate DI Adjust pH to 7.0 with NaOH then add DI to 1 liter, NaCl NaH2PO4 H2O EDTA DI Adjust pH to 7.4 with NaOH then add DI to 1 liter, Tris EDTA Adjust pH using Tris stock solution, Tris NaCl EDTA Adjust pH to 8.0 using Tris stock solution. Henderson-Hasselbalch equation. phosphate and acetate) readily form coordination complexes. And if A minus concentration over HA concentration is equal to one, that means that they have This, and its low cost, make tris one of the most common buffers in the biology/biochemistry laboratory. number from a smaller number. probably know the pK_a and you know the concentrations going to be talking mostly about this in terms of the 2 0 obj So what we're gonna do, is we're gonna rearrange this equation important relationship that is really helpful to remember. For a buffer to be most effective . p Ka: Because most biological reactions take place near-neutral pH between 6 and 8, ideal buffers would have pKa values in this region to provide maximum buffering capacity there. It is not absorbed through cell membranes, and it is essentially transparent to UV light. 1. you, but I actually find, well, (laughs) I find logs Components: MES Monohydrate(500 mM), Water(rest) Method: Prepared in 18.2 megohms-cm 1 water and filtered through 0.22-micron filter. Click on Continue. - [Voiceover] We're gonna }}h7~z1 :MK You must choose a buffer that has a pKa value near the middle of the range required (in general, it is recommended to use a buffer with a pKa value that is at least within one pH unit of the target pH). deprotonated). Has a pKa of 6.10 at 25C. acid, you wanna add some base. It may not look like it Analytical tests (DNase, RNase, protease activity, heavy metals and others upon request), 15 business days after order confirmation, Product registered under the European Chemicals Agency's REACH regulation, ADD: No.28, Huadong Road, Daliao District, Kaohsiung City, 831, Taiwan. pH: 5.5 0.15 Storage: Room Temp. that the acid has an extra H. We can rearrange the Assay (titr. Buffers work better within 1 unit of the pKa. Presented here are three common formulations (Mishkind, Sodium cacodylate buffer [Na(CH3)2 AsO2 3H2O] is a alternative to Srensens phosphate buffer. - user560 Apr 6, 2012 at 4:10 @leonardo. HEPES is a similar pH buffering compound that contains a piperazine ring. Solutions are stable at 2-8C for months. x}"tOUFC],yyTLk{'reV&@R\ ra!7_?>9472Mwt_vxga8g=r.X{?? The last scenario, pH is less than pK_a, well in that case, we're raising 10 to a negative number, because we're subtracting a bigger Keep in mind that high levels of phosphate may be somewhat toxic to plant cells (Sabatini, Add the following to create 100 ml of phosphate/citrate buffer solution. kinds of information. If you alter that the equation, by removing the p as well, then it gets kind of complex to look at but it can be done. The pH (and p Ka at ionic strength I0) of the buffer solution changes with concentration and temperature, and this effect may be predicted using online calculators. The concentration, temperature, and ionic composition . What do you call a reaction where the pH of two substances is brought closer to 7? And specifically, we're Change in pH of MES buffer with dilution in pure water? let's do a quick review of what exactly is a buffer. A lot of times, you just wanna know, you know, what's in your solution, depending on what you pKa 25C (1) 3.1 (2) 4.8 (3) 6.4 Sodium cacodylate trihydrate pKa 25C 6.3 MES monohydrate pKa 25C 6.1 ACES pKa 25C 6.8 PIPES pKa 25C 6.8 Imidazole pKa 25C 7.0 MOPSO pKa 25C 6.9 HEPES sodium pKa 25C 7.5 HEPES pKa 25C 7.5 BIS-TRIS propane pKa 25C (1) 6.8 (2) 9.0 BES pH: 6.5 0.15 Storage: 2-8C. Co., Ltd - Fine Chemicals Division. Contact with this buffer is hazardous;[5] skin or eye exposure should be cleaned well with water and medical aid should be sought in the case of eye exposure, swallowing or inhalation of dust. MES has a lower pKa than MOPS, making the MES SDS Running Buffer faster than the MOPS SDS running buffers. power is equal to one. MOPS and MES decompose when autoclaved in the presence of glucose. Not for use in diagnostic procedures. 0.2 M dibasic sodium phosphate; 0.1 M citric acid (Pearse, 1980). Direct link to dilu.ruwangika's post what happens to the buffe, Posted 4 years ago. v6P0\?@g%]?32:Q-(^:o"ch[[v9 MQ|uX}n; ~yk?flo5g`[kckKk6;OS?rk@/{Bw2v,LY`+3\,;a, S2u}V7;KCn*t--XeUJ3w5t`z[k+ o-2Iug}s#1{^wKX]O?rZ&U|epWf|LSj)}M6qSKmJ`87nw[).%\Sn8ujwaU9(O{`@vHAw]?5i:4IiovpKk%Ex rR_z+ X Why is this important for biologists? Keep buffer concentration as low as possible yet enough to maintain pH. The log in pH is left untouched. 5)You will see a message confirming the order. <> stream But before we go to the endobj It has been tested and recommended for polyacrylamide gel electrophoresis. I want to produce a 0.1 M MES buffer solution at different pH values to check for enzyme activity. https://" : " http://");document.write(unescape("%3Cspan id='cnzz_stat_icon_1263368784'%3E%3C/span%3E%3Cscript src='" + cnzz_protocol + "s13.cnzz.com/z_stat.php%3Fid%3D1263368784%26show%3Dpic' type='text/javascript'%3E%3C/script%3E")); The buffer range of MOPS is 6.5~7.9. <>/Metadata 1837 0 R/ViewerPreferences 1838 0 R>> So that gives us 10 to the pH minus pK_a is equal to A minus over HA. Mix appropriate volumes of stock and add an equal volume of distilled water to make a final 0.1 M Srensens phosphate buffer solution (Srensen, 1909; Gomori, 1955). (7) For study on electron transport and phosphorylation of chloroplast sample preparation. This is, as written, it's the acid dissociation Its chemical structure contains a morpholine ring, MES buffer has a buffer range of pH 5.8 to 6.5. The Buffer ratio increases with an increase in pH. at all the possible scenarios for these three things. Specifically, sodium acetate buffer (pKa 4.75) ranging from pH 3.75-5.75, MES buffer (pKa 6.1) from pH 5.1-7.1, PIPES buffer (pKa 6.75) from 5.75-7.75 and HEPES buffer (pKa 7.5) from 6.50 . MES is used as a buffering agent in biology and biochemistry. And as you can see up here, an acid and its conjugate base are just related by the fact In addition, the experimenter should pay attention to the sterilization of containers, water and other additives.
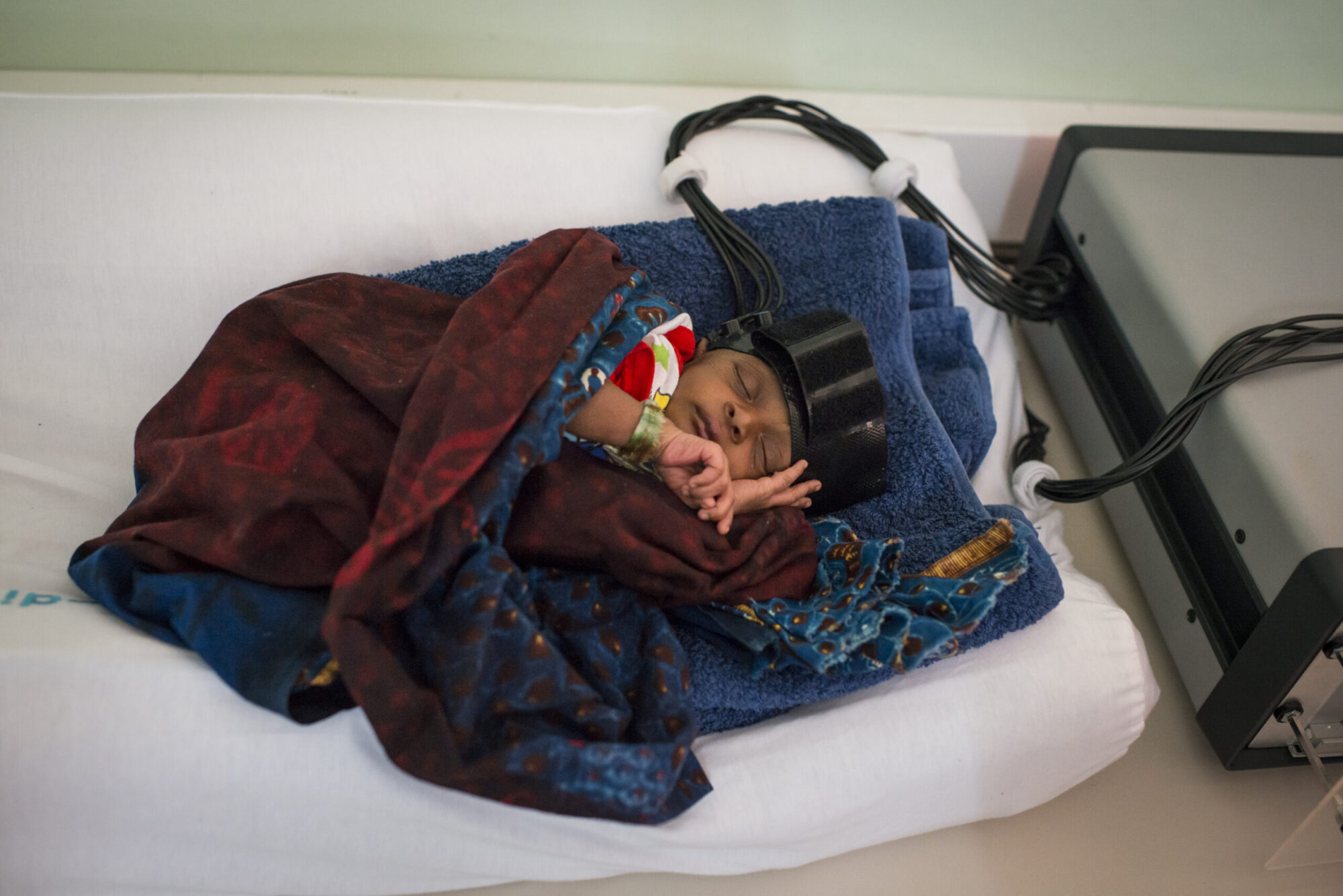
Brain Imaging for Global Health